Natural colours are considered food additives in the food and beverage world and are strictly regulated in terms of their identity, purity, the food categories in which they are allowed, and their usage rate. A recent example is the 2020 EFSA review of permitted max limits for Annatto across various food categories. It was also linked to the safety evaluation of the different types of annatto and their definition in the EU regulation of Annatto Bixin E160b(i) and Annatto Norbixin E160b(ii).
But how did they come to this decision? How are the uses and usage rates defined, and above all, how can we know that a food additive is safe for human consumption?
The answer is the ADI, or Acceptable Daily Intake, and its continuous monitoring versus dietary exposure to the additive. In this article we’ll show you how it’s calculated and how that applies to natural colours.
What is ADI?
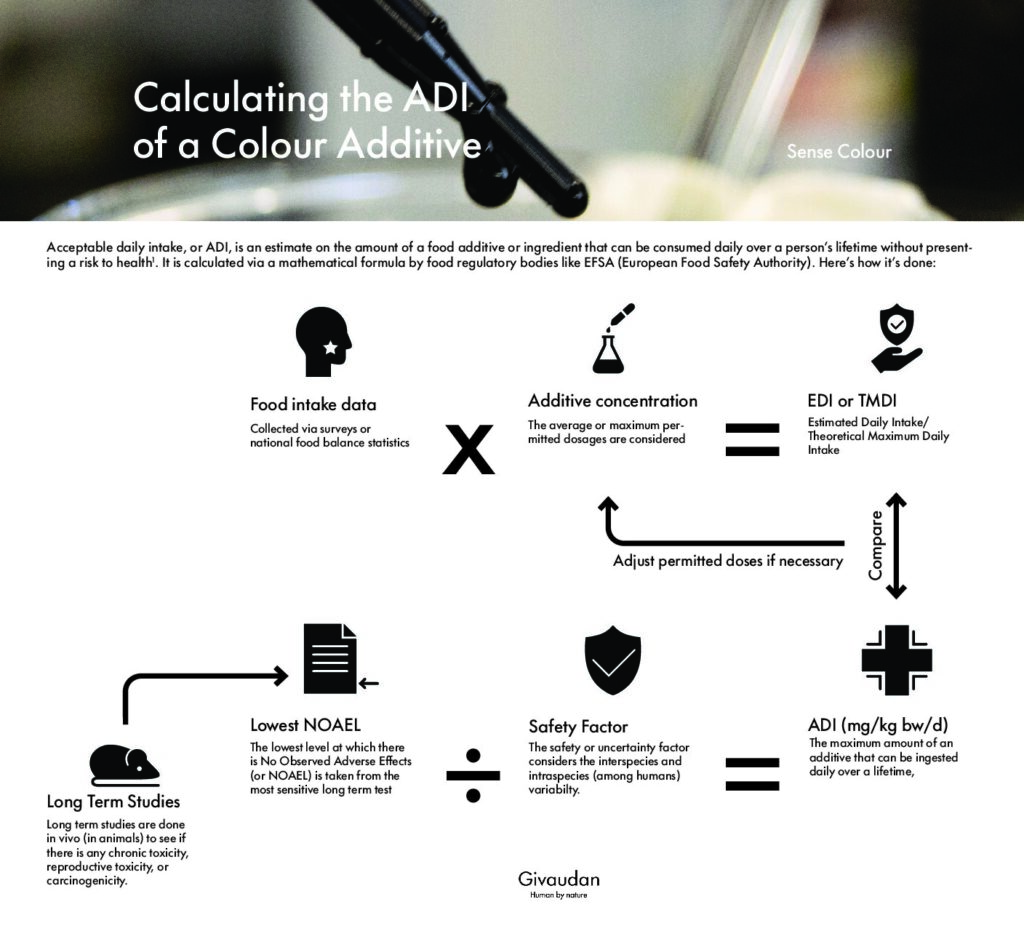
ADI is the estimation of the amount of an additive that can be ingested daily throughout a human’s lifetime without presenting an appreciable risk to health. It’s used as a reference for consumer protection and to harmonize regulatory controls, facilitating international food trade and is expressed in mg/kg body weight per day.

The Starting Point
So how is it calculated? The risk assessment of any chemical substance, including natural colour additives, begins by considering all existing scientific evidence on how the substance behaves in biological systems. Currently, the most reliable and commonly used way to gather safety information on new food additives is through in vivo tests on animal models, although other methods are being developed for future use.
These tests involve administering incremental doses of the additive orally to selected animals, allowing for the determination of a safe starting point or reference. The NOAEL (No Observed Adverse Effect Level) is typically used as that starting point, representing the highest dose of the additive that does not cause significant adverse effects compared to a control. The most sensitive species is chosen to determine the NOAEL*.
Uncertainty Factor
To extrapolate the results of animal studies to humans, and to consider the large variability in responses among individuals (e.g., children, the elderly, immunodeficient individuals), a safety or uncertainty factor is chosen. In many cases, this safety factor is 100, but it can vary depending on the availability and quality of the data obtained from in vivo tests. This safety factor divides the NOAEL to obtain the ADI:

Real Intake and Exposure
The ADI is then compared to the actual intake of the additive. This is done using estimates of average or maximum consumption of food groups containing the additive for the population or a subgroup of interest (e.g., children).
This information is collected using consumption surveys or food balance statistics. This estimate is then multiplied by the industry recommended usage rates or the current allowance. If the estimated intake is higher than the ADI for any subgroup of the population, regulatory authorities modify the permitted usage rates for the most impacting food categories.
Is NOAEL the best reference point?
The use of NOAEL as a starting point for determining the ADI has been questioned in recent years. This is because the robustness of the NOAEL depends on factors such as experimental design, dose range selection, and statistical power.
As an alternative, the concept of Benchmark Dose (BMD) has been proposed. The BMD is the concentration of the additive that produces a predetermined change in the adverse effect response (benchmark response or BMR, which is usually set at 5% (or 10%). Mathematical modeling is used to consider the dose dependency of observed effects, resulting in a confidence interval rather than a singular value. The lower limit (BMDL : Benchmark Dose Lower bound) of the confidence interval is taken as the new reference value, which is then divided by the uncertainty factor to calculate ADI.

BMDL may be slightly higher or slightly lower than NOAEL, depending on the actual experimental data, but is considered a more accurate, evidence based reference point. Although not widely used yet, BMD may become more common in ADI assessment in the future.
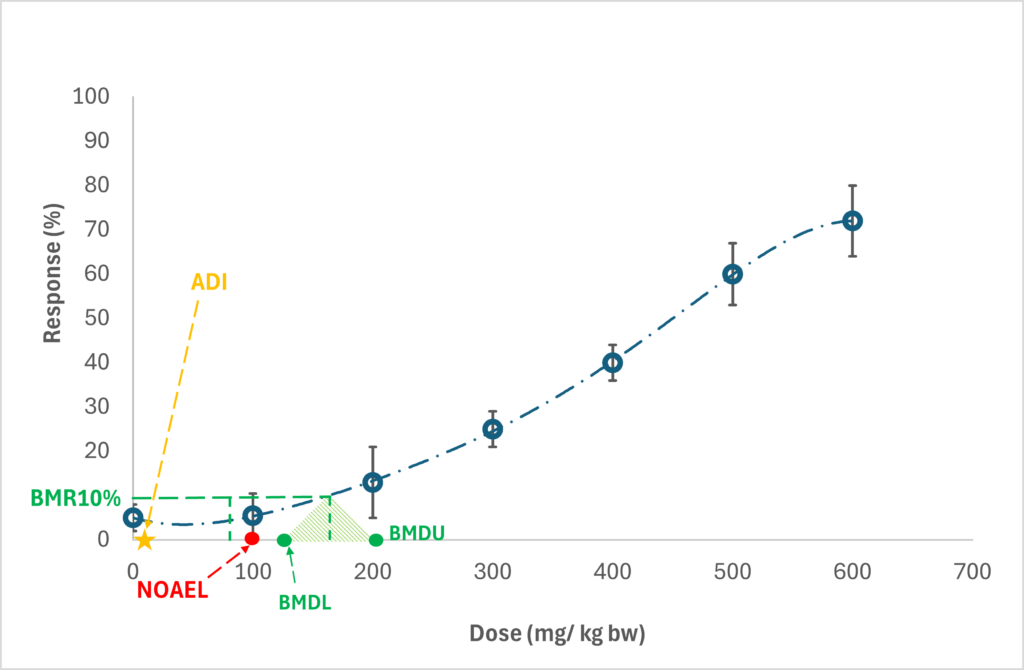
As you can see, ADI is by far a sufficiently conservative estimate of the safe levels of intake of a food additive.
Natural colour additives are carefully assessed and monitored by expert scientific committees that advise regulatory agencies all over the world to guarantee consumer protection and guide their safe and fair technological use.
*It is important to note that Givaudan Sense Colour does not conduct animal testing unless requested by a regulatory authority.