Chlorophyll is a green pigment that is naturally found in the chloroplasts in green plants. Its abundance in nature makes it ideal for use as a natural food color.
But there are several types of chlorophyll available as a food coloring and it can get confusing to figure out which is the right one for your product. Not to worry – we will walk you through the differences so you can choose the best option for your application.
Chlorophyll & Chlorophyllin
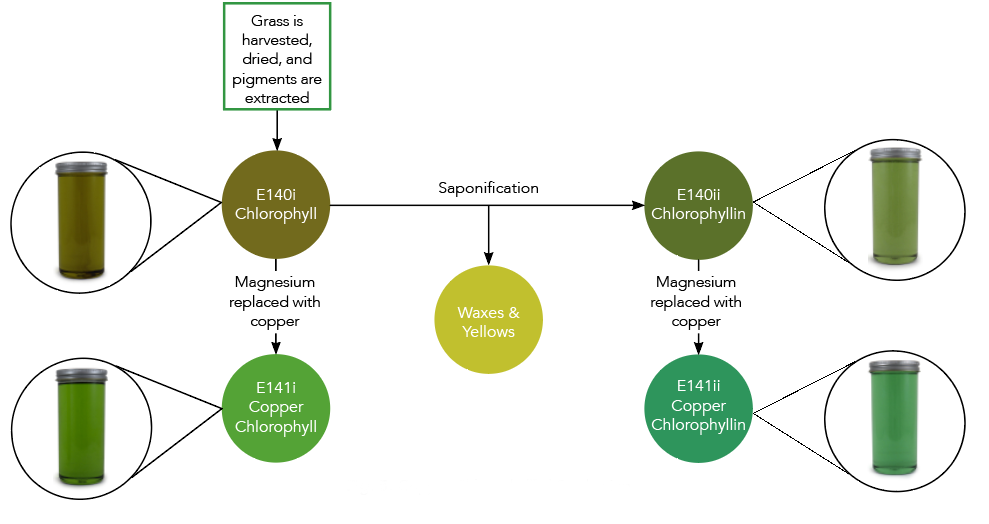
When initially extracted, the green pigment is called chlorophyll and is naturally in an oil-soluble form. It can be used in this form for oil-soluble applications like compound coatings or fat-based frostings.
However, in this original oil-soluble format, the applications it can be used in are limited. That is why it is often converted into a water-soluble form, called ‘chlorophyllin’, expanding its use to a wider variety of food and beverage applications. Chlorophyllin is simply the water-soluble version of chlorophyll.
To convert chlorophyll to chlorophyllin, it is put through a process called ‘saponification’. During this process, non-water-soluble elements such as waxes and yellow pigments, like carotenes, are removed. The resulting pigment is soluble in water and appears slightly more vibrant in color than the original chlorophyll. It can be used in applications like hard candies and boiled sweets.
To sum it up: Chlorophyll is the oil-soluble form, while chlorophyllin is the water-soluble form. Both provide green colors for different types of applications.


‘Copper’ Chlorophyll & Chlorophyllin
Even though Chlorophyllin is more vibrant than the original chlorophyll, both chlorophyll and chlorophyllin are relatively dull in color. They appear brownish-green and are very susceptible to fading in the presence of heat, light, and acid. To achieve more stable and vibrant shades of green, these colors are put through a process called ‘coppering’.
Chlorophyll naturally contains a magnesium ion, highlighted green in the image below. However, magnesium is a reactive metal that is easily displaced from the molecule when it comes in contact with things like hydrogen in citric acid, causing a loss of its green color. This reactivity can be seen in everyday activities such as when grass is cut and turns brown or when peas are overcooked and turn yellow.
To prevent this fade or color change from occurring, the magnesium ion can be replaced by a metal that is less reactive, like copper.
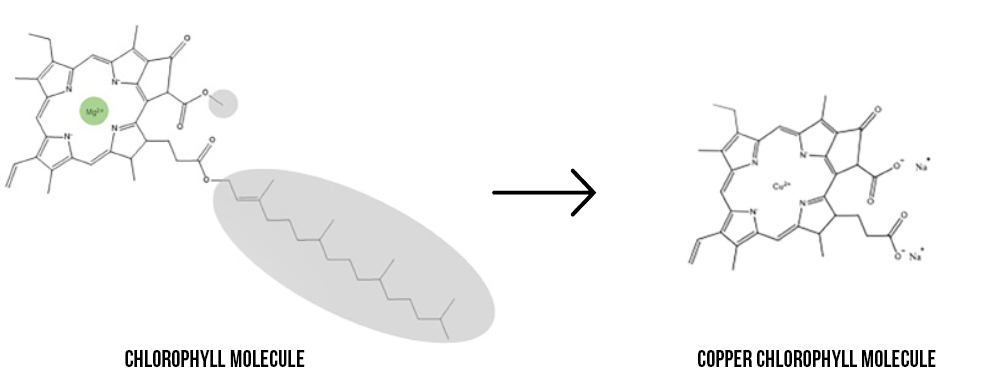
When the magnesium is replaced, the molecule is now called ‘copper chlorophyll’ (the oil soluble form) or ‘copper chlorophyllin’ (the water-soluble form). Since copper is less reactive, it will remain in the copper chlorophyll[in] molecule in acidic environments. This allows it to be used in lower pH in applications such as confections and beverages, in addition to improving heat and light stability.
Coppering also greatly improves the color. As you can see in the image below, copper chlorophyll and copper chlorophyllin are much more vibrant than their un-coppered counterparts.
Visit our Chlorophyll page to learn more! Or get started with a sample.






