What are anthocyanins?
Before we dive into why anthocyanins change color, it’s important to know what they are. Anthocyanins are natural, water soluble pigments responsible for the blue, purple, pink, and red colors in many fruit and vegetable sources. In the food coloring industry, they are typically extracted from sources such as purple corn, purple carrots, radishes, elderberries, and other fruits and vegetables that are bred specifically for their high pigment concentrations.
When used in an application with a lower pH, like confections or beverages, anthocyanins will appear bright red to pink. But when that same anthocyanin is put in an application that has a higher pH, such as a cupcake or its frosting, the anthocyanin will appear bluish-purple. But what is causing this color shift? Why can’t the anthocyanin color work the same in every application?
It all has to do with the natural reaction the molecule has to the pH of its surrounding environment.
So why do Anthocyanins Change Colors?
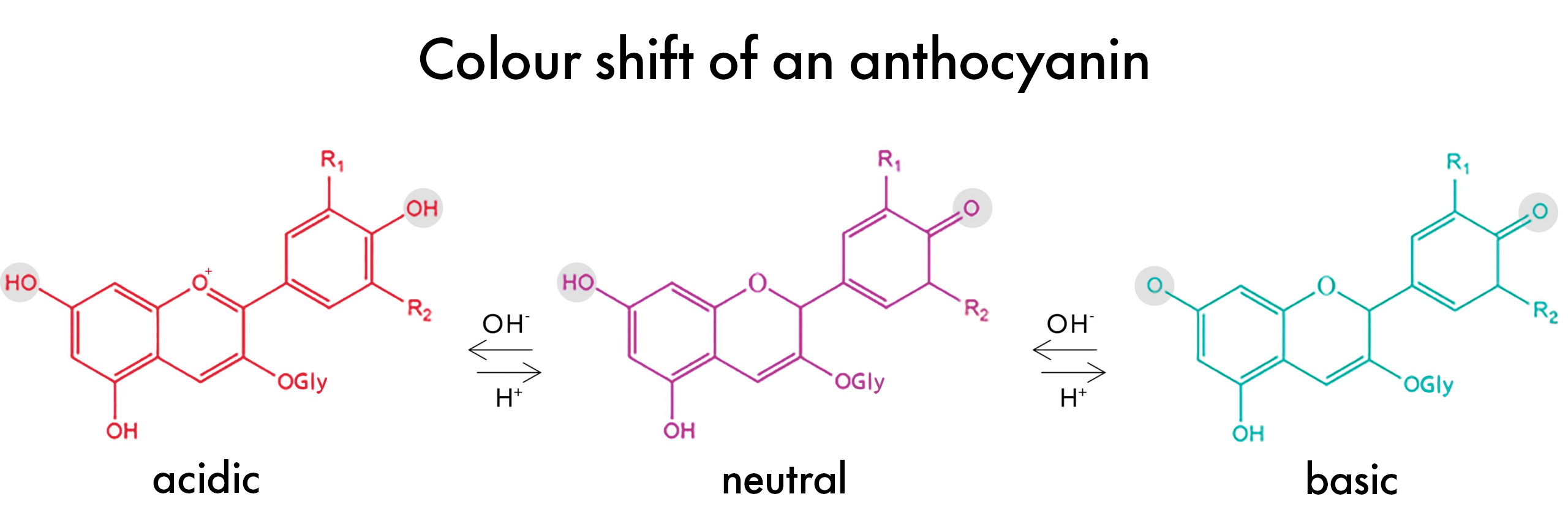
Anthocyanins change color in different pH levels because their molecular structure actually shifts as the pH of the solution they are in changes from acidic to basic and vice versa. This makes these pigments unique compared to other natural colors.
At a low pH of around 3, the anthocyanin molecule is ‘protonated’. This means the phenolic -OH groups (highlighted grey in the figure above) have hydrogens. In this environment, the anthocyanin is a positive ion, or cation. It absorbs light in the blue-green spectrum (approx. 450-560nm) and appears red to the human eye.
As the pH of the environment increases, however, the anthocyanin molecules become ‘deprotonated’ –protons are removed from the phenol groups – and the light absorption of the molecule shifts, now absorbing light in the yellow-orange spectrum (approx. 570-620nm) giving it a blue-ish purple appearance to the human eye.
Decrease the pH again, and the protons re-bond to the molecule therefore changing the light absorbance once again and reverting the color back to the original red to pink. And that’s how Anthocyanins change color…Pretty cool, right?
Want to get started with a sample? Click here.